According to the information released by the Center for Food Evaluation, State Administration for Market Regulation and the Special Food Information Query Platform, in 2024, State Administration for Market Regulation issued a total of 1390 health food (dietary supplement) registration approvals, 346 of which are new health food products.
Related Links
- China Health Food Registration and Filing
- Analysis of the Filing Status of Health Foods (Dietary Supplements) in China for 2024
- Analysis of China Health Food (Dietary Supplement) Registration in the First Half of 2024
CIRS conducted a detailed summary of these 346 new products, and analyzed from the following points:
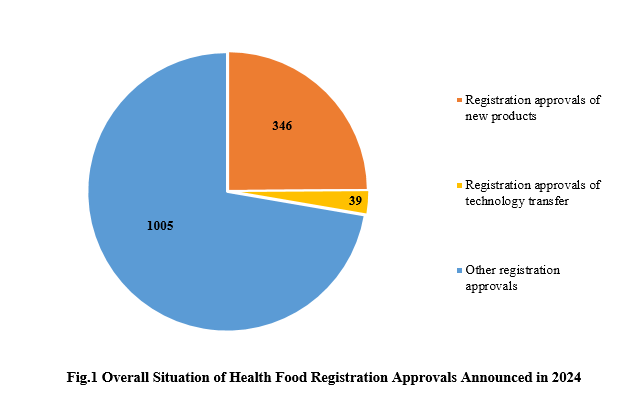
1. Health Food Registration Approvals Announced in 2024
Among the 1390 registration approvals, 346 are new products, accounting for 24.89% of the total; 39 are products for technology transfer registration, accounting for 2.81% of the total. The rest 1005 registration approvals are possibly for renewal of registration and change registration.
2. The Number of Health Food Registration Approvals Announced in Each Month
As shown in Fig.2, the total number of new products approvals and the frequency of issuance in the first half of 2024 are both higher than that in the second half.
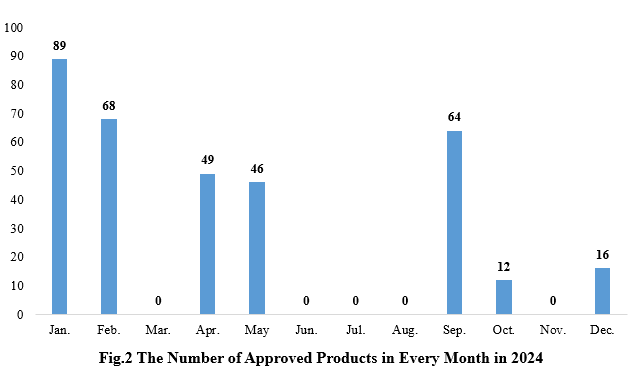
Note: There is a lag in the release of approval notice information, resulting in two products not being able to match the specific approval release date, thus the products are not included in the statistics.
3. The Number of Approved Products in Different Regions
The 346 approved new products are from 30 provinces (municipalities and/or autonomous regions). Beijing, Guangdong and Shandong occupy the top three spots with the number of 75, 44 and 28 respectively, accounting for 21.68%, 12.72% and 8.09% of the total new products.
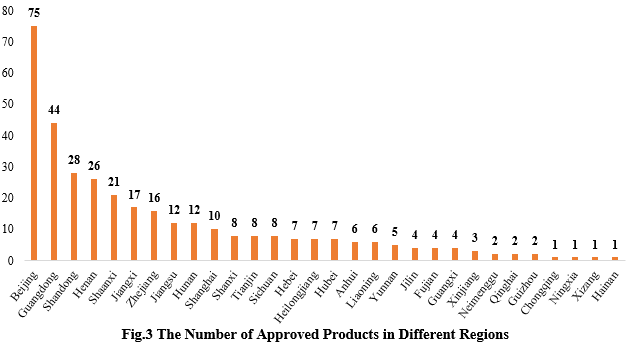
Note: If the applicant includes more than one enterprises and comes from different provinces, each province is included in the statistics.
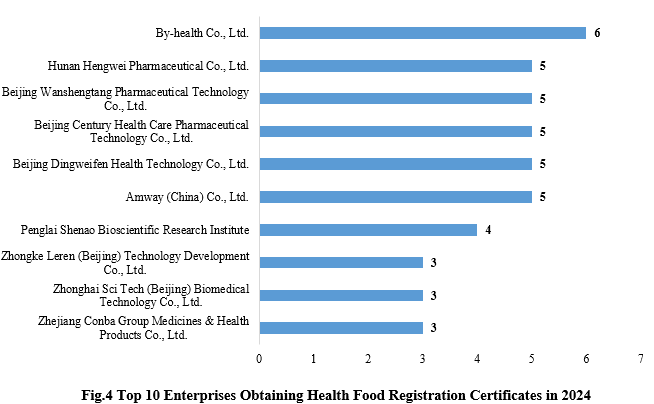
4. Enterprises Obtaining Health Food Registration Certificates
By-health Co., Ltd. obtained 6 health food registration certificates, ranked first in 2024. The top ten enterprises obtaining new registration certificates in 2024 are shown in the Fig.4.
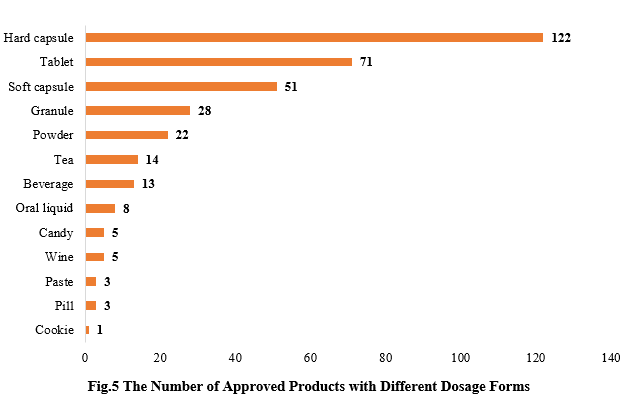
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved new products include capsule, tablet, powder, oral liquid, etc. Among them, hard capsule products received the largest number of approvals, with the number of 122, accounting for 35.26%, followed by tablet products, with the number of 71, accounting for 20.52%.
In addition to the common health food dosage forms, there are also some food forms that have received registration approvals, such as beverages, cookies and candies.
6. The Number of Approved Products with Different Health Functions
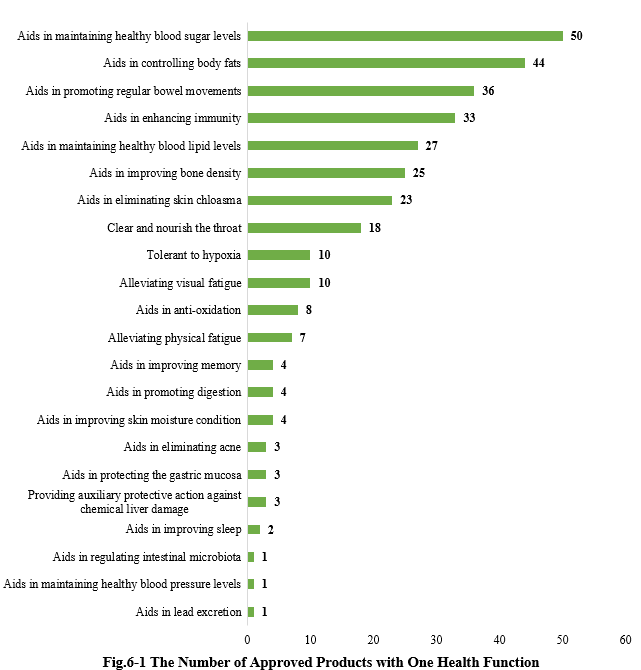
6.1 Single health function
As shown in Fig.6-1, there are totally 317 new products approved with the single health function, among them, products with the health function of “Aids in maintaining healthy blood sugar levels” received the largest number of approvals, with the number of 50.
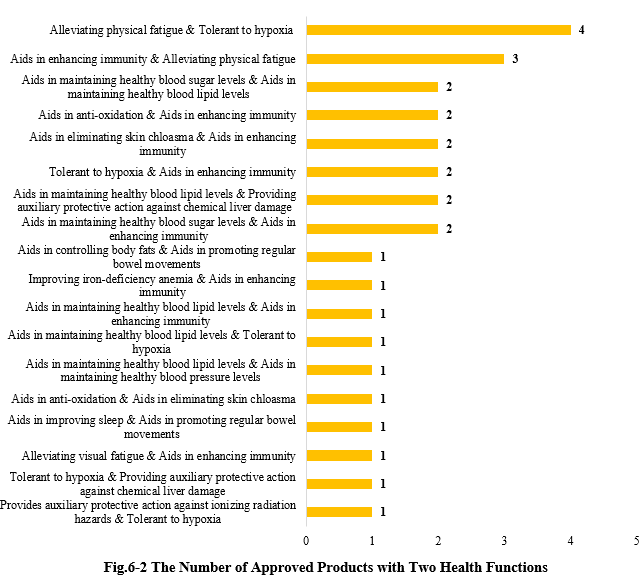
6.2 Two health functions
Except for those new products with single health function, there are also 29 products approved with two health functions (shown in Fig.6-2). And 4 products with the function combination of “Alleviating physical fatigue & Tolerant to hypoxia” got registration certificates in 2024.
7. CIRS Comments
It can be seen that nowadays the dosage form and function of health food products are closer to the daily life, the consumers tend to be younger as well. Enterprises are suggested to complete enough market researches during the R&D stages, so that the latest health demands can be mastered.
The SAMR officially issued the Key Points for the Review of the Change of Registration Certificate for Health Foods with No Validity Period and No Technical Requirements in Production and Sale on November 1, 2024, giving a start to the certification replacement work.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Note: There may be a lag in the data release of the Special Food Information Query Platform. The data in this article is only for reference, and the actual situation is subject to the official announcement.
Data Source:
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.

