As of December 31, 2023, the State Administration for Market Regulation (SAMR) issued a total of 1,456 health food (dietary supplement) registration approvals, 749 of which are for new health food products. CIRS Group has conducted a detailed summary and analyzed them from the following perspectives.
Related Links
Analysis on the Filing Status of Health Food (Dietary Supplement) in 2023 in China
Analysis on Health Food and Dietary Supplement Product Registrations in China in 2022
Overview of Health Food Registration Approvals Issued in 2023
Among the 1,456 registration approvals, 749 are new products, accounting for 51.44% of the total. The other 707 registration approvals fall into the categories of registration renewal, change of registration, and technology transfer registration.

Number of Health Food Registrations (by Approval Time)
As shown in Fig.2, the registration approvals for new products were released in March, May, June, July, September, and December of 2023, with 147, 101, 74, 36, 139, and 252 respectively. No such approvals were released in the remaining months.

Number of Approved Products in Different Regions
The 749 approved new health food products are from 29 provinces (municipalities and/or autonomous regions). Beijing, Guangdong, and Zhejiang secure the top three spots with the number reaching 181, 100, and 57, accounting for 24.17%, 13.35%, and 7.61% of the total, respectively. Details are as follows:

Note: If two applicants from different regions have applied for the same new product, each region is counted separately in the statistics.
Enterprises Obtaining Health Food Registration Certificates
Sirio Pharma Co., Ltd. and Beijing Century Health Care Pharmaceutical Technology Co., Ltd. demonstrated outstanding performance in 2023, each securing 15 approvals for new product registrations, jointly holding the top position. Beijing Wanshengtang Pharmaceutical Technology Co., Ltd. and Beijing Jinpingkang Pharmaceutical Technology Co., Ltd. both obtained 12 new product registration approvals, sharing the second position. Zhonghai Kechuang (Beijing) Biomedical Technology Co., Ltd. secured 9 approvals, ranking third.
The top ten enterprises obtaining new registration certificates in 2023 are shown in Fig.4.

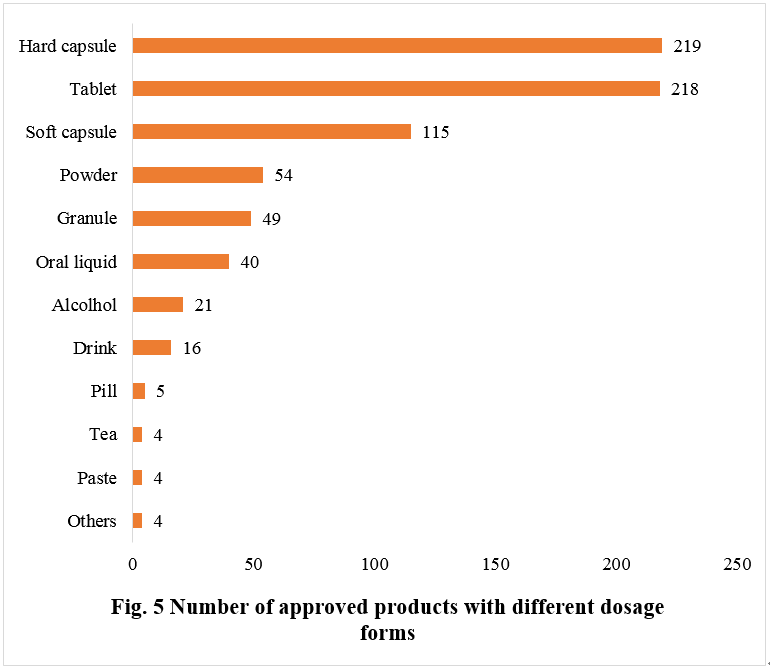
Number of Approved Products with Different Dosage Forms
Among the approved new health food products in 2023, the number of products in the form of hard capsules and tablets is quite close, with 219 and 218 respectively, ranking first and second, accounting for 29.24% and 29.11% of the total, respectively.
Number of Approved Products with Different Health Functions
Single health function
As shown in Figure 6-1, among the new product approvals issued in 2023, there are a total of 701 applying for a single health function. Among them, the health function of aiding in enhancing immunity has the most applications, totaling 255, accounting for 36.38% of the category. It’s worth mentioning that 7 nutrition supplements received registration approvals in 2023, including 5 calcium supplements, 1 supplement for calcium, iron, zinc, and vitamin C, and 1 supplement for calcium and magnesium.
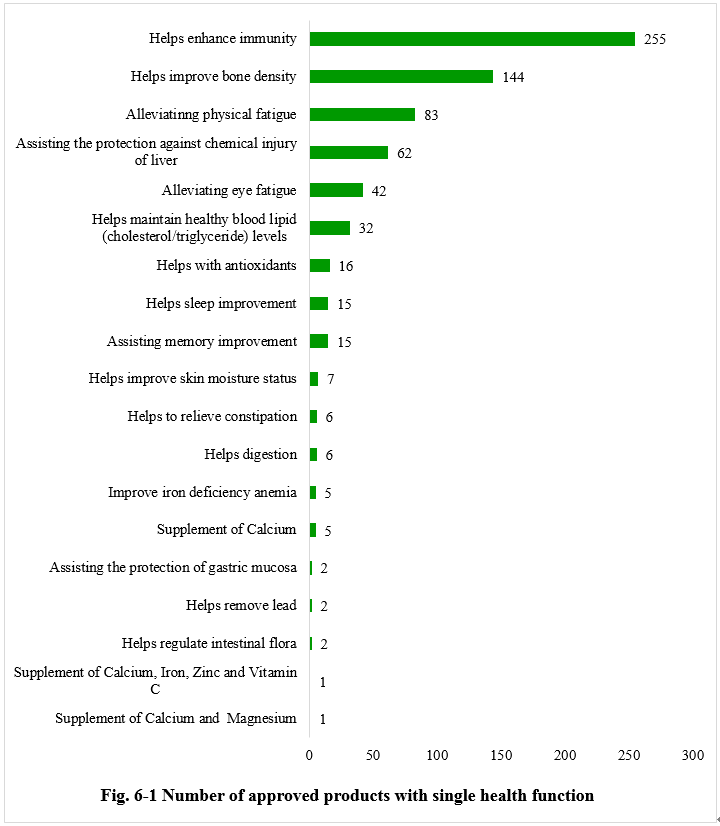
Note: Due to the lack of information in the Special Food Information Query Platform, the specific health function of three products cannot be found, thus these three products are not included in the above statistics.
Two health functions
As shown in Fig.6-2, 45 products applied for two health functions in the total new product approvals in 2023. The predominant category was health food claiming enhancing immunity and relieving physical fatigue, totaling 18 products, accounting for 40% of this category.

CIRS Comments
Compared to 2022, there has been a remarkable 178% increase in the approval of new health food products in 2023. This signals an accelerating pace in the review process for registered health food products, indicating a gradual rebound in the health food registration market. In August 2023, a series of regulations, including the Guideline on Testing and Evaluation of Health Food Functions (2023 Edition) and Detailed Rules for Technical Evaluation of New Functions and Products of Health Food (Trial) were successively implemented. Since then, the registration of new health food products has finally gained momentum, breaking free from the constraints of the current Directory of Health Food Functions, which is anticipated to inject a fresh surge of energy into the health food market. CIRS Group has launched ChinaFoodDB, a one-stop query platform for food raw materials and regulations launched by CIRS Group, aiming to provide enterprises and users with front-end compliance services, including food raw material inquiries, formula compliance self-checks, and access to various regulations and official announcements. It is believed to become a powerful tool for enterprises in the process of formula R&D and product innovation.
Note: 1. The data in this article is sourced from the Special Food Information Query Platform.
2. The release of data on the Special Food Information Query Platform may be subject to delays. The data in this article is for reference only, please refer to the official information.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

