Excepted for GMP, do you know other GXP System? The list of GXP as following:
1. GXP list
| GXP | English Name | Chinese Name |
|---|---|---|
| GLP | Good Laboratory Practice (unimplemented) | 医疗器械非临床研究质量管理规范 (未实施) |
| GCP | Good Clinical Practice | 医疗器械临床试验质量管理规范 |
| CMP | Good Manufacturing Practice | 医疗器械生产质量管理规范 |
| GSP | Good Supply Practice | 医疗器械经营质量管理规范 |
| GUP | Good Use Practice | 医疗器械使用质量管理规范 |
2. The Action Stage of GXP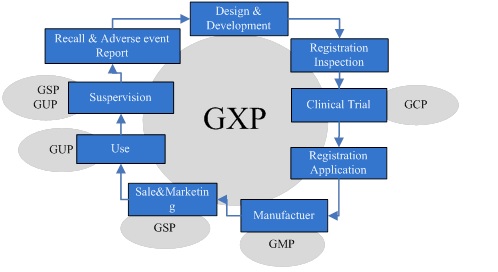
GXP | Implementation time |
GCP: | |
Good clinical practice for medical device | Exposure Draft |
Technical guideline for clinical evaluation of medical device | 2014.09.11 |
Technical guideline for clinical evaluation of In Vitro Diagnostic Reagent | 2015.05.19 |
| GMP: | |
| Good Manufacturing Practice for medical device | 2015.03.01 |
| Good Manufacturing Practice for Medical Devices Appendix for Sterile Medical Devices | 2015.10.01 |
| Good Manufacturing Practice for Medical Devices Appendix for Implantable Medical Devices | 2015.10.01 |
| Good Manufacturing Practice for Medical Devices Appendix for In Vitro Diagnosis Reagents | 2015.10.01 |
| Guideline for Medical Device Good Manufacturing Practice On-site Inspection | 2015.09.25 |
| Guideline for Medical Device Good Manufacturing Practice On-site Inspection for Sterile Medical Devices | 2015.09.25 |
| Guideline for Medical Device Good Manufacturing Practice On-site Inspection for Implantable Medical Devices | 2015.09.25 |
| Guideline for Medical Device Good Manufacturing Practice On-site Inspection for In Vitro Diagnosis Reagents | 2015.09.25 |
| REMARKS: 1.All Class III medical device manufacturers shall conform to these rules before 2016.01.01; 2. All medical device and in vitro diagnostic reagent manufacturer shall conform to those rules before 2018.01 | |
| GSP: | |
| Good Supply Practice for Medical Device | 2014.12.12 |
| Guideline for Medical Device Good Supply Practice On-site Inspection | 2015.10.15 |
| GUP: | |
| Administrative Measures for Quality Supervision on the Use of Medical Devices | 2016.02.01 |
4. Supervising pattern of GXP
| GXP | Object of supervision | Supervising pattern | Supervising subject |
|---|---|---|---|
| GCP | Clinical trial institutions | 1. On-site Inspection | Food and Drug Administration |
| GMP | Manufacturers | ||
| GSP | Manufacturers and Distributors | ||
| GUP | Medical institutions: Hospital, Beauty salon, etc. |
5. How to execute the GXP?
| How |
| GCP: |
| The medical device with Practicing License of Medical Institution or qualification of Grade 2A and above can apply for GCP License according to Determination Measurement of Qualification of Medical Device Clinical Trial Institutions (Exposure Draft) |
| GMP: |
| Pursuant to the specified clauses of Guideline for Medical Device Good Manufacturing Practice On-site Inspection: 1.The result of inspection is disqualification if the clauses with “*”1 are not conformed to; 2.The result of inspection is disqualification if the clause without “*”2 which can affect the quality of product are not conformed to. 3.The result of inspection is rectification and re-inspection if the clause without “*”2 which cannot affect the quality of product are not conformed to. |
| GSP: |
| In according with the specified clauses of Guideline for Medical Device Good Supply Practice On-site Inspection: 1.The result of inspection is disqualification if the clauses with “※”1 are not conformed to; 2.The result of inspection is disqualification if discord rate of the clause without “※”2 are larger than 10%; 3.The result of inspection is rectifications within a time limit if discord rate of the clause without “※”2 are less than 10%; |
| GUP: |
| In according with the requirements of Medical Device Good Use Practice including purchasing, storage, use and maintaining, etc. |
| REMARKS: 1.The clause with “*”or “※” refers to key item; 2.The clause without “*” or “※” refers to general item;The number of clauses without “※” not conformed to/( Total number of clauses without “※”—Total number of clause without “※” which is inapplicable )*100% |

