
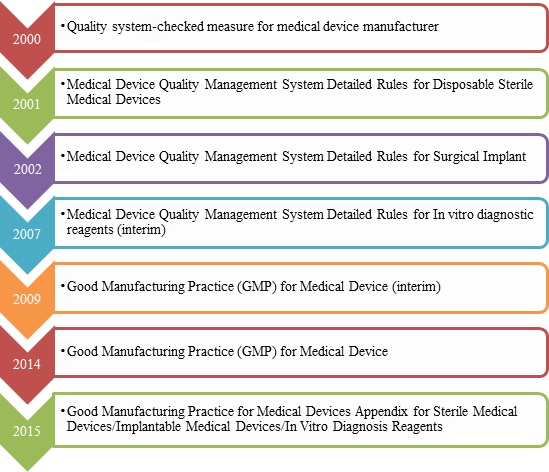
Dec 29th, 2014, CFDA issued the Good Manufacturing Practice for Medical Device to strengthen the supervision and management of medical device manufacturing and standardize quality management. Whereafter, CFDA issued three Appendixes (Sterile Medical Devices, Implantable Medical Devices, In Vitro Diagnosis Reagents) for Good Manufacturing Practice for Medical Devices on Jul 17th, 2015.
Those GMP rules specify relevant requirements on organization and personnel, premises and facilities, equipment, document management, design and development, procurement, production management, quality control, sales and after-sales services, control of nonconforming products, adverse event monitoring, analysis and improvement etc. And the medical device manufactures shall set up and improve the quality management system in accordance with those GMP rules.
1.GMP for medical device Revision History
2.The key points of GMP
2.1 Organization & personnel
The qualification requirements and responsibility & authority for top management, management representative, department head, professionals and operators, etc.
Note:
Personnel who are engaged in implantable animal medical device and allogeneic medical device shall have trainings in special technology, safeguard and precautious measures according to their product and operation. The personnel include cleaning staff, service staff and so on.
Note:
Personnel who are engaged in implantable animal medical device and allogeneic medical device shall have trainings in special technology, safeguard and precautious measures according to their product and operation. The personnel include cleaning staff, service staff and so on.
2.2 Premises and facilities
Premises and facilities shall be designed, composed and used rationally according to the product’s characteristic, technological process and corresponding requirements of cleanliness grades. And manufacturer shall equip with inspection sites and facilities which shall be conform to the production scale, variety and inspection requirements.
2.3 Equipment
Manufacturer shall equip with production equipment and technological equipment which meet the product and scale to ensure the production can be operated effectively, as well as inspection instruments and equipment which adapt to product inspection with clear operational specifications.
2.4 Document management
Manufacturer shall establish quality management system files, including quality policy, quality objective, quality manual, procedures files, technical documents and other documents required. Technical documents shall include product technical requirements and relevant standards, technological procedures, operation instruction, inspection and testing operation instruction, installment and service operation instruction, and so on.
2.5 Design and development
Design and development output shall meet the requirement of input, including related information of purchasing, production and service, and product technical requirements, etc.
If the change of materials, accessories or product function will make an influence on medical device safety and effectiveness, the enterprise shall make a risk evaluation for this change, and shall take measures to reduce the risk to the acceptable levels if necessary, and shall meet the requirements of related regulations for medical device.
If the change of materials, accessories or product function will make an influence on medical device safety and effectiveness, the enterprise shall make a risk evaluation for this change, and shall take measures to reduce the risk to the acceptable levels if necessary, and shall meet the requirements of related regulations for medical device.
2.6 Procurement
Manufacturer shall establish purchasing controls procedures to ensure that all purchased or otherwise received product and services conform to specified requirements, which cannot less than the relevant requirements of the national laws and mandatory standards. Purchasing data shall be traceable.
Manufacturer shall evaluate and select potential suppliers, contractors, and consultants on the basis of their ability to meet specified requirements, including quality requirements. The evaluation shall be documented.
Manufacturer shall evaluate and select potential suppliers, contractors, and consultants on the basis of their ability to meet specified requirements, including quality requirements. The evaluation shall be documented.
2.7 Production management
Manufacturer shall establish quality management system to ensure the product meet the requirements of mandatory standards and product technical requirements.
Manufacturer shall compile technological procedure, standard operation procedure, and so on to define the key process and special procedures.
All the information of production shall be traceable.
Manufacturer shall compile technological procedure, standard operation procedure, and so on to define the key process and special procedures.
All the information of production shall be traceable.
2.8 Quality control
Manufacturer shall establish the procedures for quality control to ensure the special requirements for inspection department, personnel, etc. what is more, manufacturer shall compile the inspection procedure according to mandatory standards and product technical requirement and provide the inspection report or certificate.
2.9 Sales and after-sales services
Manufacturer shall establish the sale record and which meet the requirement of traceability.
2.10 Control of nonconforming products
Manufacturer shall identify, record, isolate and evaluate the defective products, and take appropriate measures to deal with defective products based on the evaluation results.
2.11 Adverse event monitoring, analysis and improvement
Manufacturer shall establish adverse events monitoring system of medical device in accordance with the relevant regulations, to carry out monitoring of adverse events and re-evaluation, and keep relevant records. For medical devices with existence of security risk, manufacturer shall carry out the recall in accordance with relevant regulations, and report to the authorities.
3. Common problems during GMP inspection
- Lack of quality personnel to conduct required material reviews and make disposition decisions;
- Lack of training and evaluation for special positions;
- Environment and facilities noncompliance; grads-design of pressure difference unreasonable;
- Design and development files do not correlate to application dossier for registration;
- Purchasing control confusion, and lack of quality certificate of raw materials;
- Lack of management measures for nonconforming products.
If you need free Good Manufacturing Practice for Medical Device--Guiding principle of on-site inspection for implantable medical devices (EN version), you can contact us for a free!

