
On April 3, 2025, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued a preliminary opinion (SCCS/1677/25) on Hydroxyapatite (nano) (CAS No. 1306-06-5). The deadline for comments is set for May 30, 2025.
Preliminary Opinion on Hydroxyapatite (nano)
The SCCS concludes the following:
Based on the data provided, the SCCS considers hydroxyapatite (nano) safe when used at concentrations up to 29.5% in toothpaste, and up to 10% in mouthwash.
This conclusion is based on the available evidence, which shows that hydroxyapatite (nano) does not pose a mutagenic hazard, cytotoxicity, or inflammatory effects even when tested at high concentrations in a buccal mucosa cell model. Any uptake of hydroxyapatite (nano) by buccal mucosa is considered negligible, and the epithelial cells with internalized particles will be shed out over time as they are continually replaced. Also, any unintentionally ingested HAP nanoparticles during the use of oral-care products will undergo rapid dissolution in the gastric fluid and therefore do not raise any nano-specific concern over safety.
This safety evaluation only applies to the hydroxyapatite (nano) that has the following characteristics:
- composed of rod-shaped particles of which at least 87% (in particle number) have aspect ratios equal to or less than 3, and the remaining 13% have aspect ratios not exceeding 9;
- the HAP particles are not coated or surface-modified.
Regulatory Requirements Interpretation of Hydroxyapatite (nano) in China
The Chinese Cosmetic Ingredient Regulatory Database (ChinaCosIng), independently developed by CIRS Group, indicates that Hydroxyapatite (nano) (CAS No. 1306-06-5) is used for abrasive, bulking, oral care, or skin conditioning in cosmetics.

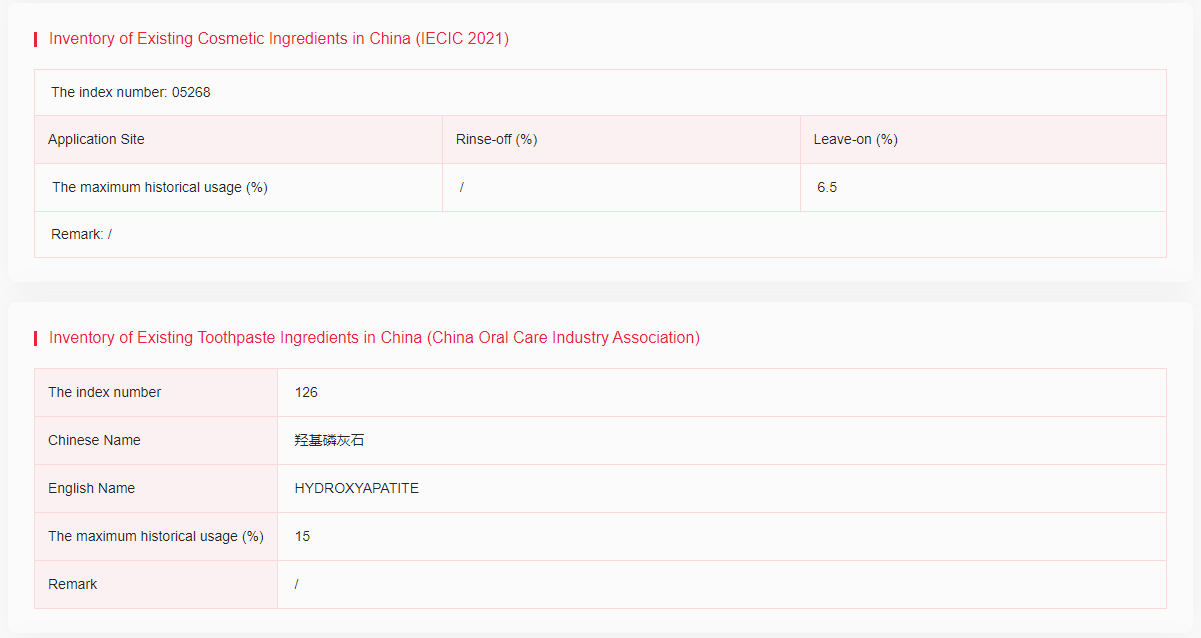
Hydroxyapatite (nano) has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC 2021), with the maximum historical usage in leave-on products of 6.5% and the Inventory of Existing Toothpaste Ingredients in China (China Oral Care Industry Association) with the maximum historical usage of 15%.
About CIRS
The CIRS cosmetic team is dedicated to ensuring that cosmetic products meet stringent global regulatory standards. It can provide one-stop services covering the whole life-cycle of a personal care product, which includes cosmetic ingredient development, physical/chemical tests, toxicological tests (in vivo & in vitro), efficacy studies (in vivo & in vitro), ingredient registration, and product registration.
Cosmetic services in China:
- China Cosmetic Registration and Filing;
- China New Cosmetic Ingredient Registration and Filing;
- China Cosmetic Ingredient Quality and Safety Information Code Application (NMPA Code);
- China Cosmetics Safety and Efficacy Test;
- China Cosmetic Safety Assessment Report;
- China Toothpaste Filing;
- China Disinfectant Notification; and
- China Cosmetic Formula/Label/Claim Review
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

