The Good Clinical Practice for Medical Device (GCP) will put in force on Jun 1st, 2016. In order to strengthen the supervision of medical device clinical trial, CFDA released On-site Audit Procedure of Medical Device Clinical Trials which is the supportive document of GCP for public opinion on Apr 12th, 2016.
The on-site inspection procedure of medical device clinical trials is made up of seven stages, which are as following:
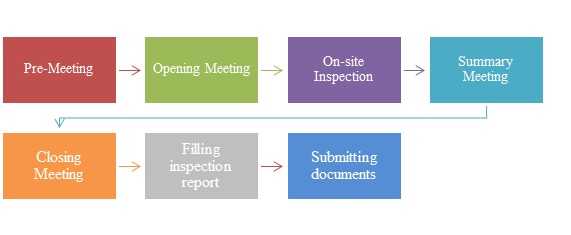
The on-site inspection procedure of medical device clinical trials is made up of seven stages, which are as following:

1. Pre-meeting
Inspection group leader shall organize the pre-meeting for all inspectors to be acquainted with inspection mission, determine the inspection mode, and realize the relevant discipline requirements prior to on-site inspection.
2. Opening meeting
Inspection group shall present the inspection notice to clinical trial institutions, and inform the staff composition of inspection group, the origin of inspection, on-site inspection discipline and requirements, and rights and obligations of clinical trial institutions.
3. On-site inspection
Inspector shall record the situation of on-site inspection completely, really and objectively while retrieving the clinical trial protocol, clinical trial report, case report form and other original clinical trial data which are preserved by clinical trial institutions. The record shall include inspection time, site and discoverable problems, and so on. Inspector can communicate with clinical trial administrative department or clinical trial researchers to realize the conditions. If it is necessary to obtain evidence, inspector can adopt different methods to save evidence, such as copy, tape, shoot, and so on.
The time for inspection shall be determined follow the principle of enough to check up on problem. Generally, inspection shall be ended in the scheduled time, and if need to extend the time, shall report and be agreed.
The time for inspection shall be determined follow the principle of enough to check up on problem. Generally, inspection shall be ended in the scheduled time, and if need to extend the time, shall report and be agreed.
4. Summary meeting
Group leader shall convoke the summary meeting, and inspectors report the problems which are found in on-site inspection. Inspection group will discuss and determine together, fill the Summary of Medical Device Clinical Trial Inspection faithfully and clearly, and verify the evident.
5. Closing meeting
Inspection group shall inform the inspection situation to clinical trial institution. And clinical trial institution shall make explain for the problem, sign and seal on related documents, and so on.
Summary of Medical Device Clinical Trial Inspection shall be signed by all staff of inspection group, observer, person in charge of clinical trial institution (or other consignor), executor, and sealed of clinical trial institution. If clinical trial institution or executor dissent the Summary of Medical Device Clinical Trial Inspection, can make an explanation and demonstration in written with signature and official seal. If clinical trial institution or executor refuse to sign, inspection group shall record and describe the circumstances.
Summary of Medical Device Clinical Trial Inspection shall be signed by all staff of inspection group, observer, person in charge of clinical trial institution (or other consignor), executor, and sealed of clinical trial institution. If clinical trial institution or executor dissent the Summary of Medical Device Clinical Trial Inspection, can make an explanation and demonstration in written with signature and official seal. If clinical trial institution or executor refuse to sign, inspection group shall record and describe the circumstances.
6. Filling inspection report
Inspection group shall fill the Medical Device Clinical Trial Inspection Report in according with on-site inspection record and closing meeting circumstances. All staff of inspection group and observers shall sign the report.
7. Submitting documents
Inspection group shall submit the Summary of Medical Device Clinical Trial Inspection, the Medical Device Clinical Trial Inspection Report and other inspection documents after inspection.

