Infant and Young Children Food Regulatory Compliance Service in China
Background
As a kind of high risk food, infant and young children food has been highly concerned by government and society, and the quality control and supervision by Chinese government become stricter in recent years. The new food safety law stipulates that infant formula food should be filed under local FDA, and the formula of infant milk powder should be registered under CFDA.
What is infant and young children food?
Infant and young children food in this article refers to infant formula food, older infants and young children formula, canned complementary foods for infants and young children, cereal-based complementary foods for infants and young children, and complementary nutritional supplements.
Related laws, regulations and national standards(Please click regulation names to download)
Product Regulatory compliance procedures
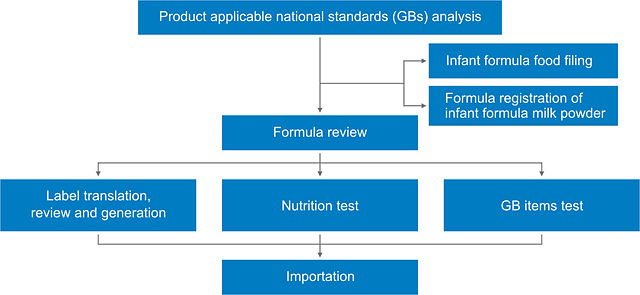
Our services
Infant and young children food applicable Chinese national standards (GBs) analysis
- Infant and young children food formula (raw materials and food additives) review
- Infant and young children food Chinese label translation, review and generation
- Infant and young children food nutrition test
- Infant and young children food GB test
- Infant and young children formula food recording
- Infant formula milk powder formula registration
- Other customized services
| S.N. | Items | Services |
| 1 | Product national standards (GBs) analysis | Evaluate the product category and find an applicable Chinese national standards (GBs) based on the formula, production process and etc. |
| 2 | l Formula (raw materials and food additives) review |
I. Analyze if all food raw materials and food additives are allowed to be used in this product. II. Analyze if the dosages of all ingredients are compliant. |
| 3 |
Chinese label translation, review or generation |
1. Label translation Translate the original information to Chinese information. 2. Label review I.Check if the Chinese label provided by client is compliant in China. II. Provide modification and risk-aversion suggestions. 3. Label generation I. Translate necessary information on original label to Chinese. II. Generate Chinese label according to GB 13432-2013 and other related standards, such as add mandatory content or remove risky content. III.Check the label to make sure it is compliant, and provide risk-aversion suggestions. |
| 4 | Nutrition testing |
I. Test on protein, fat, carbohydrate, sodium and energy (4+1). II. Test on added nutrition enhancers and other nutrients if there is additional content claim on the label. III.Nutrition test reports should be provided to CIQ during inspection declaration. |
| 5 | GB test |
I. Test on items stipulated in related products national standards (GBs), including sensory index, physicochemical index, microorganism index and etc. II. For infant formula milk powder, GB test reports should be provided to CIQ during inspection declaration. |
| 6 | Infant formula food filing (No detailed regulations for the moment) | Collect required documents and apply for the filing of food raw materials, food additives, food formula, label and etc. under local CFDAs. |
| 7 | Infant formula milk powder formula registration (Draft regulation for the moment) | Collect and write required documents and apply for the formula registration under CFDA. |
| 8 | Others | Ad-hoc consulting service and etc. |
--- What are the required materials?
| S.N. | Materials |
| 1 | Registration application |
| 2 | Formula research report and production process specification |
| 3 | Product testing report |
| 4 | Evidentiary materials of the production, research and testing capabilities |
| 5 | Other materials to prove the scientificity and safety of the formula |
--- The Registration Procedures

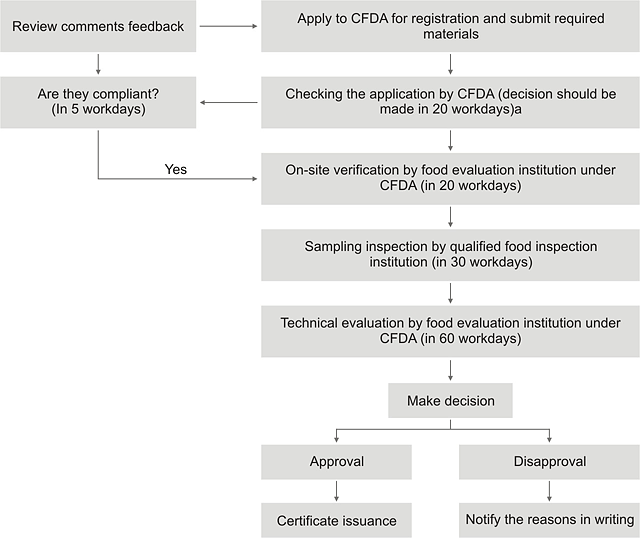
P.S.“a”: The time of on-site verification, sampling inspection and technical evaluation is not included in the 20 workdays.
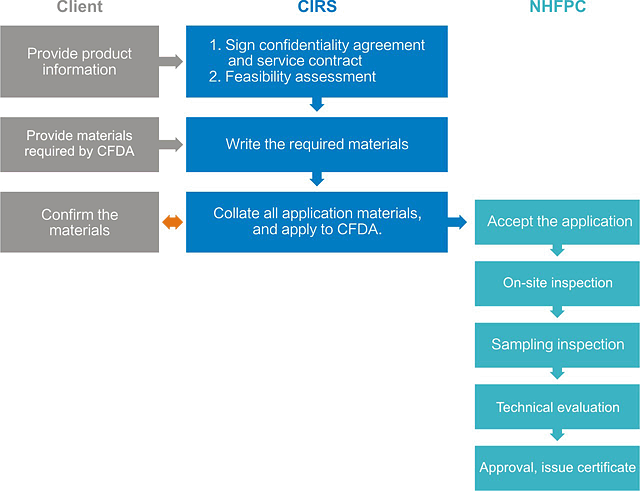

Contact us
Ms. Alice Yang, Food Safety and Regulatory Affairs Department, CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel: +86 571 8971 6579 | Fax: +86 571 8720 6533
Email: Alice.Yang@cirs-group.com






